Abstract
Ruxolitinib (RUX) is beneficial in myelofibrosis (MF) and polycythemia vera (PV) patients (pts). However, little is known about patient adherence (ADH) to this chronic therapy. Poor ADH may increase treatment failure, with serious welfare and economic consequences (Osterberg L, N Engl J Med 2005). The Adherence to Refills and Medications Scale (ARMS) (Kripalani S, Value Health 2009) and the Distress Thermometer and Problem List (DTPL) (Donovan KA, Psycho-oncology 2014) measure drug ADH and psychological distress, respectively.
After IRB approval, the RAMP (Rux Adherence in MF and PV) multicenter prospective observational study included 189 consecutive pts with MF (n. 141, 75%) or PV (n.48, 25%), that received RUX according to routine practice in 9 Hematology Centers. The ARMS included 12 questions, 6 evaluating intentional and 6 unintentional ADH. The DT describes pts’ level of distress using a scale of 0 (no distress) to 10 (extreme distress). Also, pts can select problems from a list (39 areas divided in 5 subgroups). First tests administration occurred at the earliest convenient time (week, wk0), irrespective of the time of RUX start, and again after 4 (wk4), 8 (wk8), 12 (wk12), 24 (wk24) and 48 weeks (wk48).
The aims of the RAMP study are to evaluate: 1) baseline level of RUX ADH and distress, and characteristics associated to low ADH; 2) modifications of ARMS/DTPL scores over time; 3) impact of ARMS/DTPL scores on IWG-MRT defined spleen response in MF.
Between June 2020 and March 2022, 189 pts completed ≥1 ARMS and DTPL test; median age, 70 yrs (33-89); 59.2% males. The first questionnaire was completed within 1 yr from RUX start in 127 pts (67.2%) (EARLY-ADH), and after >1 yr of RUX therapy in 62 pts (32.8%).
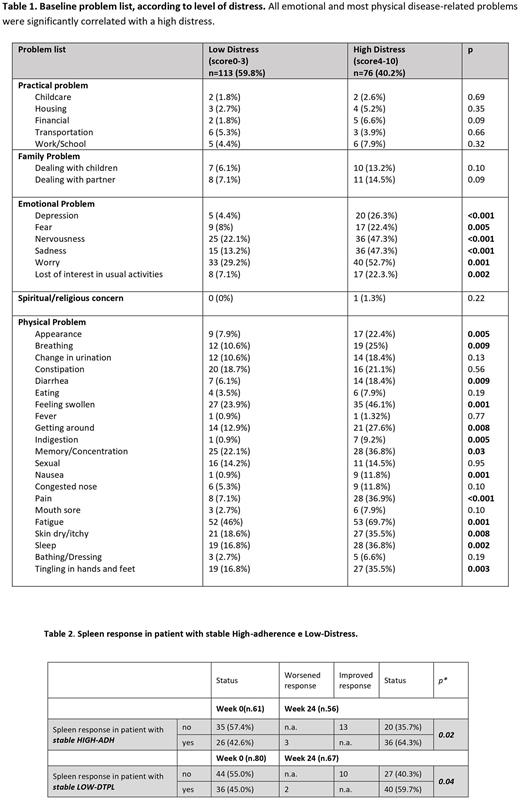
At wk0, the mean ARMS and DT scores were 13.2 (SD, 1.95) and 2.9 (SD, 2.79), respectively. Based on the DT ≥4 cutoff, 76 pts (38.1%) had high distress (Table 1). Overall, 11.1%, 4.5%, 28.5% and 55.9% of pts had no emotional/physical problems, only emotional, only physical and both problems, respectively. 77.8% of pts with both emotional/physical problems also had high distress (compared to 41.5% in pts with low distress, p=0.002).
Overall, 95 pts (50.3%) had HIGH-ADH (ARMS score of ≥14) and 94 (49.7%) had LOW-ADH (score <14). LOW-ADH was intentional in 49 (52.1%), unintentional in 20 (21.3%) and both in 25 pts (26.6%). LOW-ADH was comparable in PV (45.8%) and MF (51.8%) pts (p=0.48); however, unintentional LOW-ADH was more frequent in PV than in MF pts (43.8% vs 25.5%, p=0.02).
In multivariate regression analysis, LOW-ADH was associated to male sex (odd ratio, OR: 3.2, p=0.007), high distress (OR: 3.7, p=0.003), and RUX duration >1yr (OR: 2.23, p=0.003). Male sex and high distress remained associated with LOW-ADH in MF pts (OR:3.7p=0.002; OR: 3.6, p=0.002 respectively) and only in those who completed the ARMS/DTPL > 1 yr after RUX start (OR: 3.7, p=0.002; OR: 3.7, p=0.003, respectively).
Overall, 146 (77.2%) pts completed both tests at w24. Full completers with HIGH-ADH tended to increase over time (from 47.3% at wk0 to 52.4% at wk24), while pts with high distress decreased (from 44.3% to 36.2%). Pts with high distress more frequently also showed LOW-ADH (p=0.05). Over time, 25% of pts with baseline LOW-ADH acquired a HIGH-ADH, while distress improved in 27% of pts.
MF pts who maintained high ADH and low distress over time were more likely to obtain or maintain spleen response at 24 weeks (Mc Nemar test, p=0.02 and p=0.04) (Table 2).
At wk24, pts reporting sexual dysfunctions (13.7% vs 7.5%, p=0.04) and insomnia (24.6% vs 15.1%, p=0.01) significantly decreased compared to wk0, along with emotional distress (60.9% vs 29.5%, p<0.001).
ARMS/DTPL results at wk48 will also be provided to explore long-term ADH/distress.
The RAMP study highlights that LOW-ADH to RUX remains an unmet clinical need in MF and PV. The association between LOW-ADH and spleen response supports that ADH evaluation in real-life practice may improve MF therapy and prognosis. LOW-ADH is more frequently intentional in MF, and unintentional in PV; thus, differentiated corrective interventions are needed in the two diseases. Male pts and those on RUX for >1 yr are at higher risk for LOW-ADH and should be monitored more closely. As ADH increased over time, it is possible that periodic ARMS administration may be beneficial in itself. Since high distress correlates with LOW-ADH, DTPL evaluation and targeted support may also improve treatment success.
Disclosures
Palandri:Sobi: Consultancy, Honoraria; Sierra Oncology: Consultancy, Honoraria; AOP: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Kartos/Telios: Consultancy, Honoraria; CTI: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Iurlo:Novartis, BMS, Celgene, Incyte, Pfizer: Honoraria. Abruzzese:BMS, Incyte, Novartis, Pfizer: Consultancy. Cavo:AbbVie, Amgen, Bristol Myers Squibb/Celgene, Pfizer, GlaxoSmithKline, Sanofi, Roche, Takeda: Consultancy, Honoraria; Janssen: Honoraria, Speakers Bureau. Heidel:Novartis: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding; CTI: Consultancy, Research Funding; AOP: Consultancy. Palumbo:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal